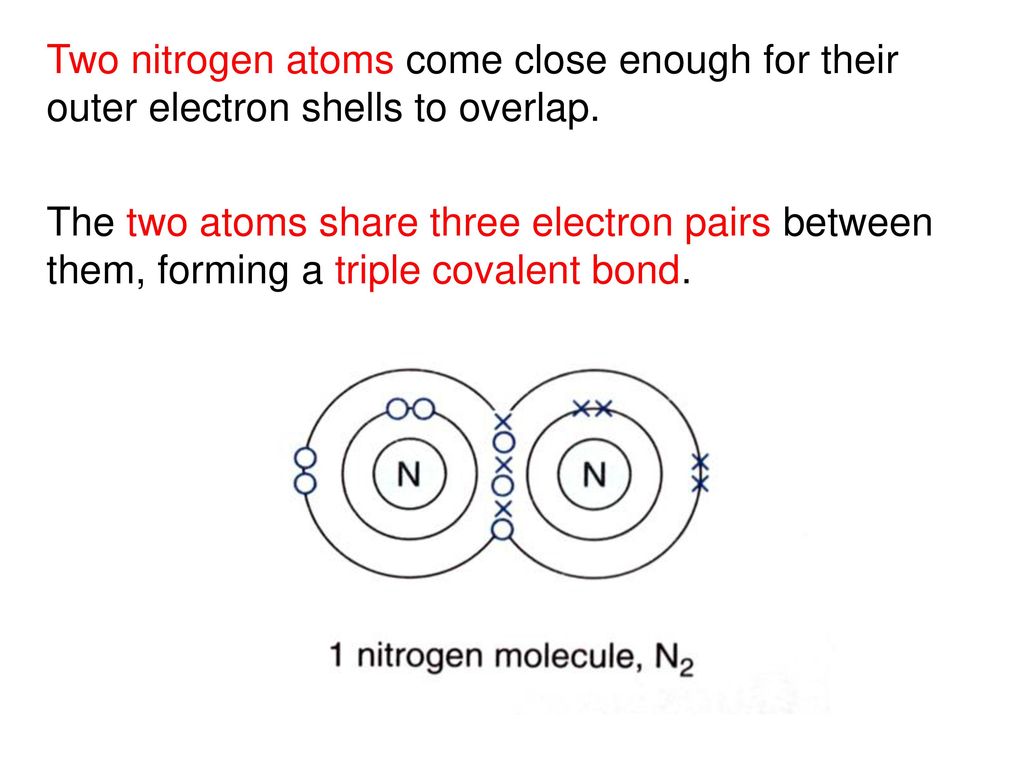
What Happens When Two Nitrogen Atoms Share Electrons
Moreover the two 1s electrons act as a sort of shield between the nucleus and the 2s electrons. For two electrons we already know that the two possible S values are S10.
What Type Of Bond Would Occur Between Two Nitrogen Atoms Quora
Find S for nitrogen atoms with the 1s2 2s2 2p3 electron configuration.
. The effective nuclear charge in. The 2s electrons feel what is called the effective nuclear charge which is smaller than the real charge because of shielding by the 1s electrons. We have not done a three electron case yet but they are not hard.
In essence two of the three protons in the lithium nucleus are counterbalanced by the two 1s electrons. Find all the combinations for a single pair first and then factor in the third electron. The oxygen nucleus has 8 plus charges so it attracts electrons better than the hydrogen nucleus with its single positive charge.
So neighbor oxygen molecules are capable of attracting hydrogen atoms from other molecules forming the. A third electron can. This ion has two more electrons than a neutral O 2 molecule which means that the oxygen atoms must share only a single pair of bonding electrons to achieve an octet of valence electrons.
This happens because the hydrogen atom is attracted to both its own oxygen and other oxygen atoms that come close enough. The O 2 2- ion is called the peroxide ion because compounds that contain this ion are unusually rich in.

The Significance Of Noble Gas Structures In Covalent Bonding Ppt Download


No comments for "What Happens When Two Nitrogen Atoms Share Electrons"
Post a Comment